Self-assembly of rare octanuclear quad(double-stranded) cluster helicates showing slow magnetic relaxation and the magnetocaloric effect†
Abstract
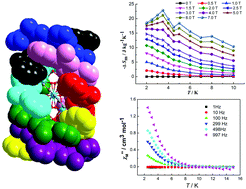
Four rare quad(double-strand) cluster helicates containing Ln8 polyhedron cores, namely [Ln8(HL)4L4(OH)6(H2O)2(acac)2](ClO4)4·2CH3OH·12H2O (Ln = Gd for 1, Tb for 2, Dy for 3, and Ho for 4, respectively), have been assembled by using lanthanide salts and flexible ligands of H2L obtained from a 1 : 2 condensation reaction between 1,1′-(2,6-pyridyl)bis-1,3-butanedione and 2-aminophenol. In each complex, four pairs of organic ligands (HL− and L2−) wrap the Ln8 polyhedron presenting a bicapped face-sharing double cubane configuration in a propeller-like form, resulting in the formation of octanuclear quad(double-stranded) helicates by coordination interactions of Ln–O/N bonds. The dc magnetic susceptibility measurements of complex 1 suggested the existence of weak antiferromagnetic couplings between neighboring GdIII ions and the magnetocaloric effect with −ΔSmaxm = 22.9 J kg−1 K−1 at 3.5 K for ΔH = 7 T. Ac magnetic susceptibility measurements of complex 3 at zero dc field demonstrated a slow magnetic relaxation behavior below 8.0 K with an effective energy barrier of 2.14 K.


 Please wait while we load your content...
Please wait while we load your content...