Morphological evolution of hollow NiCo2O4 microspheres and their high pseudocapacitance contribution for Li/Na-ion battery anodes†
Abstract
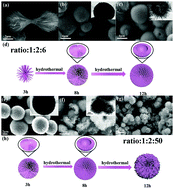
Hollow urchin-like NiCo2O4 microspheres (∼3 μm) with a large specific surface area (158.57 m2 g−1) have been synthesized by a facile template-free hydrothermal method and a morphology evolution mechanism of “bundles-solid spheres-hollow urchin-like microspheres” was proposed. The hollow urchin-like structure appears when the hydrothermal time is increased to 8 h, which can be accelerated by the addition of excess urea. Benefiting from the unique three-dimensional (3D) hollow structure and the desired composition, the NiCo2O4 microspheres exhibit an excellent reversible specific capacity for lithium ion batteries (991 mA h g−1 after 50 cycles) and sodium ion batteries (322.3 mA h g−1 after 50 cycles). The unique 3D hollow structure offers enough space to alleviate volume expansion caused by the Li+/Na+ insertion/extraction, and the perfect electrical conductivity of spinel binary metal oxides facilitates the transport of ions and electrons. A high capacitance contribution of 90% was achieved for LIBs at 0.3 mV s−1, while the capacitance contributions for SIBs were only 36% at 0.3 mV s−1 and 73% even at 5 mV s−1, which indicates that a capacitive-controlled charge storage mechanism plays a dominant role in the Li+ storage of NiCo2O4 microspheres. This work has guiding significance in the preparation of electrode materials with high electrochemical performance.


 Please wait while we load your content...
Please wait while we load your content...