Kinetics of manganese leaching from an iron-rich manganese dioxide ore with bagasse pith as a reductant†
Abstract
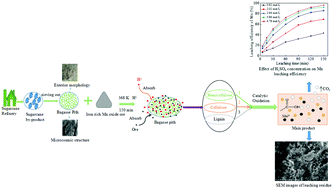
The kinetics of reductive leaching of manganese from iron-rich manganese dioxide ore (IRMDO) using bagasse pith as a reductant in the presence of sulfuric acid was investigated. The effects of reductant type, stirring speed, ore particle size, leaching temperature, sulfuric acid concentration and weight ratio of IRMDO to bagasse pith on the leaching efficiency of manganese were studied. The experimental results indicated that an increase in temperature, sulphuric acid concentration and dosage of bagasse pith significantly enhanced the leaching efficiency of manganese, but manganese leaching efficiency increased with the decrease of the particle size of the ore. A leaching efficiency of 97% for total Mn was obtained under the following optimized conditions: 700 rpm of stirring rate, 10 : 5 of IRMDO to bagasse pith weight ratio, 4.78 mol L−1 of sulfuric acid concentration, 368 K of leaching temperature and 150 min of reaction time. The leaching efficiency of manganese based on the shrinking core model was found to be controlled by a surface chemical reaction. The apparent activation energy of the chemical reaction was 50.53 kJ mol−1, while the reaction was found to be of 0.94 and 0.36 order in terms of sulfuric acid and bagasse pith concentration, respectively. In addition, the decomposition mechanism of bagasse pith was investigated. The microstructures of the manganese oxide ores in pre- and post-leaching reactions were further studied by SEM and XRD analysis.


 Please wait while we load your content...
Please wait while we load your content...