PhI(OAc)2/NaX-mediated halogenation providing access to valuable synthons 3-haloindole derivatives†
Abstract
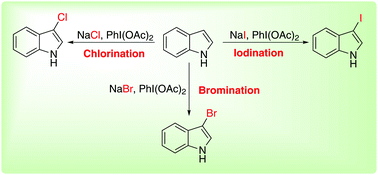
This paper describes a mild phenyliodine diacetate mediated method for selective chlorination, bromination, and iodination of indole C–H bonds using sodium halide as a source for analogous halogenations. The combination of NaX and phenyliodine diacetate provides an invincible system for halogenation of indoles. This protocol was compatible with a wide array of indole substrates and provides straight forward access to potential halogenated arenes.


 Please wait while we load your content...
Please wait while we load your content...