Water-soluble superbulky (η6-p-cymene) ruthenium(ii) amine: an active catalyst in the oxidative homocoupling of arylboronic acids and the hydration of organonitriles†
Abstract
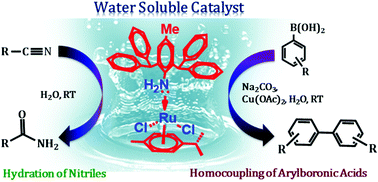
A phosphine free water-soluble superbulky amine–ruthenium–arene complex (2) encompassing 2,6-bis(diphenylmethyl)-4-methylaniline was synthesised in good yield. 2 was characterized by FT-IR, 1H NMR, and 13C NMR spectroscopies, TGA and elemental analyses. The structure of 2 was confirmed by a single-crystal X-ray diffraction study. The ruthenium centre in 2 adopts the pseudo-octahedral geometry due to the η6-p-cymene ring and bulky aniline ligand along with two chloro groups. Besides, complex 2 was efficaciously employed as a catalyst in the hydration of organonitriles to amides. This reaction proceeds efficiently for a wide range of substrates in an environmentally benign medium and is an economically reasonable synthetic route to amides in good yields. In addition, 2 acts as an excellent catalyst in the oxidative homocoupling of arylboronic acids in water. A range of arylboronic acids undergo a homocoupling reaction in the presence of catalyst 2 to yield symmetrical biaryls in reasonable to good yields.


 Please wait while we load your content...
Please wait while we load your content...