The linear relationship derived from the deposition potential of Pb–Ln alloy and atomic radius†
Abstract
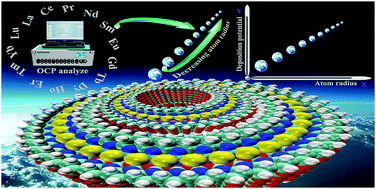
Lanthanides possess extremely similar properties because their radius only changes slightly due to lanthanide contraction. The atomic radius and ionic valence, as the structural features of the atom, have far-reaching effects on properties of the lanthanide metal, alloying or covalent compounds. We found that regularity exists with atomic radius and physicochemical properties, such as electronegativity, atomic volume, and coefficient of thermal expansion, which gives us some inspiration: there may be a correlation between the deposition potential and atomic radius. Therefore, in this paper, the chemical and electrochemical properties of intermetallic compounds involving lead chloride (PbCl2) and lanthanide metals (La, Ce, Pr, Gd and Dy) at 873 K using the same substrates (Mo) as working electrodes are discussed. We discovered a linear relationship that exists between the atomic radius and deposition potentials by summarizing the experiment data. We further investigated the application of the mathematical equation in the temperature range of 873–1023 K to verify the universal applicability of the line equation.


 Please wait while we load your content...
Please wait while we load your content...