Metal–organic framework sorbents for the removal of perfluorinated compounds in an aqueous environment†
Abstract
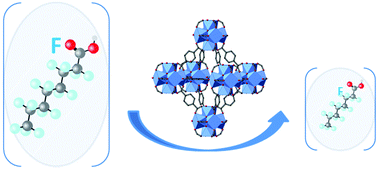
Special attention is paid to synthetic anionic surfactants, particularly the perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) anions (denoted as PFOX), due to their persistence in aquatic environments and the high toxicity of these surfactants. Two metal organic frameworks (MOFs) based on Zr-oxo clusters, UiO-66 and the equivalent perfluorinated UiO-66-(F4), were prepared and tuned for the selective adsorption of PFOA and PFOS from an aqueous solution. The MOFs have been characterized by powder X-ray diffraction, thermogravimetric analysis, infra-red spectroscopy, and BET measurement and their efficiency for the uptake of PFOX has been determined. The influence of several parameters such as the kinetics, the m/V ratio or the temperature on the adsorption capacity was investigated. These two porous MOFs have been revealed to be excellent materials for the removal of these pollutants with saturation sorption capacities of around 470 mg g−1 under some conditions. The fluorinated MOF cavity of UiO-66-(F4) has been revealed to increase the affinity for the sorption of fluorinated pollutants due to the fluorine–fluorine interactions without changing drastically the saturation capacity compared to UiO-66. These results indicate that Zr-MOFs provide a promising platform for the removal of fluorinated organic pollutants from aqueous solutions.


 Please wait while we load your content...
Please wait while we load your content...