Nanoparticles based on lipidyl-β-cyclodextrins: synthesis, characterization, and experimental and computational biophysical studies for encapsulation of atazanavir†
Abstract
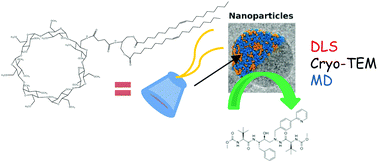
Amphiphilic cyclodextrins were synthesized from permethylated βCD with the aim of forming nanoparticles (NPs) that would encapsulate specific molecules (e.g. drugs) which could enhance their otherwise poor bioavailability. By grafting different fatty acids, four amphiphilic CDs were obtained. The self-assembling properties of three of these compounds were evaluated demonstrating micromolar critical aggregation concentration (CAC). Additionally, the stability of these nanoparticles was studied revealing that the compounds with C18 chains could be stored at 4 °C for prolonged periods without any issue. Finally, reliable characterization of NPs made of di-oleoyl-glycerolipidyl-β-cyclodextrin (DOCD) was performed by combining DLS, cryo-transmission electron microscopy (cryo-TEM) and molecular dynamics (MD) simulations. This revealed that DOCD nano-assemblies are roughly nano-scaled, spherical objects (diameter ca. 120 nm) without internal organization or aqueous compartments. Finally, atazanavir, used as a model drug, was entrapped in NPs whilst MD simulations were used to investigate molecule entrapment. This revealed that atazanavir interacts with DOCD to form a drug-loaded NP which does not fit with the 2 : 1 stoichiometry encapsulation classically observed in the cyclodextrin cavity.


 Please wait while we load your content...
Please wait while we load your content...