High capacity MoO3/rGO nanocomposite anode for lithium ion batteries: an intuition into the conversion mechanism of MoO3†
Abstract
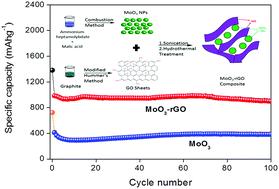
MoO3 is a potential anode material for Li-ion batteries (LIBs) because of its high theoretical capacity (1117 mA h g−1). The major hurdles in realizing this high capacity are its low conductivity and large volume variations during intercalation/de-intercalation processes. To mitigate these shortcomings, we have synthesized reduced graphene oxide (rGO) wrapped MoO3 nanoparticles (NPs). This involves the synthesis of MoO3 NPs as the first step and then subjecting the synthesized MoO3 NPs to hydrothermal treatment along with graphene oxide (GO) sheets to form rGO wrapped MoO3 NPs. Electrochemical impedance spectra show that a 13% MoO3/rGO nanocomposite has the least conductive resistance among the different nanocomposites. Several physicochemical characterization techniques have been used to confirm the desired state of the obtained material. Ex-XRD studies were carried out to inspect the mechanism of MoO3 and found that it initially follows a simple lithiation/delithiation mechanism and later it adopts a conversion mechanism. The new architecture exhibits an excellent electrochemical performance by displaying a high first specific discharge capacity value (984 mA h g−1) and remarkable stability (901 mA h g−1 even after 100 cycles).


 Please wait while we load your content...
Please wait while we load your content...