Fluorescence, FTIR and 1H NMR studies of the inclusion complexes of the painkiller lornoxicam with β-, γ-cyclodextrins and their hydroxy propyl derivatives in aqueous solutions at different pHs and in the solid state†
Abstract
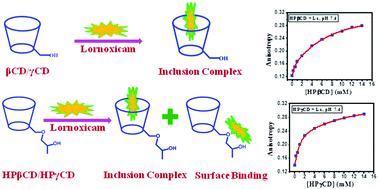
Natural cyclodextrins (CDs) and their synthetic derivatives have been studied extensively with the aim to develop good drug delivery vehicles. To be delivery vehicles, CDs need to form inclusion complexes with drugs. Here, we have characterized the complexation of lornoxicam (Lx), a drug belonging to the oxicam group of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), with different cyclodextrins (β-CD, γ-CD, HPβCD and HPγCD) in aqueous solutions at two different pHs, a physiological pH of 7.4 and an acidic pH of 2.5 to replicate the conditions in the stomach for oral administration. Steady state fluorescence spectroscopy and anisotropy studies showed that at both pHs, Lx forms host–guest inclusion complexes with different CDs, with higher binding constants at pH 2.5 than at pH 7.4. Compared to β-CD and HPβCD, γ-CD and HPγCD show greater binding constants. Structural characterization using 1H NMR and FTIR in the solid state show that predominantly the π-electron rich pyridine ring of Lx penetrates into the host cavities with deeper penetration in γ-CD and HPγCD compared to their β-counterparts. Even though Lx forms inclusion complexes with all CDs, for their hydroxy propyl derivatives, the drug can also interact with the outer surface of the CD molecules mainly near the hydroxy propyl group.


 Please wait while we load your content...
Please wait while we load your content...