Ethanol-mediated N-formylation of amines with CO2/H2 over cobalt catalysts†
Abstract
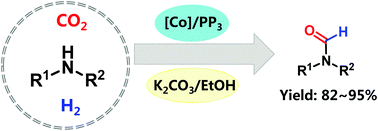
The CO2-involved synthesis of chemicals is of great significance from a green and sustainable point of view. Herein, we present an efficient Co-based catalytic system composed of a commercially available Co salt, the tetradentate phosphine ligand P-(CH2CH2PPh2)3, and a base, denoted as [Co]/PP3/base, for the synthesis of formamides via the formylation of amines with CO2/H2. It was indicated that the selectivity of products (i.e., formamide or methylamine) could be tuned to some extent via changing the solvent and the base. Using ethanol as the solvent, the Co(ClO4)2·6H2O/PP3/K2CO3 system showed high activity for the production of formamides, affording product yields of 82–95%, together with its broad substrate scope. Exploration of the reaction mechanism indicated that formamide was formed with HCOOH as the intermediate, while the methylamine byproduct was produced with HCHO as the intermediate via the hydrogenolysis of dialkylaminomethane.


 Please wait while we load your content...
Please wait while we load your content...