Gas phase dehydration of glycerol to acrolein on an amino siloxane-functionalized MCM-41 supported Wells–Dawson type H6P2W18O62 catalyst
Abstract
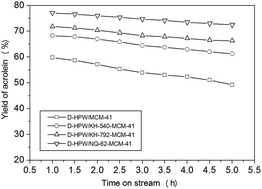
A novel amino siloxane-functionalized MCM-41 supported Wells–Dawson type H6P2W18O62 (D-HPW) catalyst was synthesized by a two-step method and investigated in the gas phase dehydration of glycerol to acrolein. The amino siloxanes (KH-540, KH-792, and NQ-62) were firstly loaded on MCM-41, and then D-HPW was impregnated. After loading of amino siloxanes, the heteropolyanions interacted with the amine groups, generating strongly distorted heteropolyanions on the surface of the host materials. As a result, these heteropolyanions with a highly deformed structure might render H+ more active, finally showing more Brønsted acid sites. The strong chemical interaction between the amine groups and the D-HPW molecules was strengthened with the increase in the number of amine groups. The results showed that the acrolein selectivity was enhanced after loading of amino siloxanes because of the increased Brønsted acid sites. The catalyst stability was enhanced, because the amount of coke decreased, and the enhancement of the interaction between the –NH2 groups in the amino siloxane and the D-HPW molecules prevented the leaching of D-HPW. Finally, D-HPW/NQ-62-MCM-41 exhibited the highest acrolein selectivity and stability. The reaction pathway for the gas-phase dehydration of glycerol on the amino siloxane-functionalized MCM-41 supported D-HPW catalyst is also proposed.


 Please wait while we load your content...
Please wait while we load your content...