Synthesis, characterization and optimization of heterogeneous catalytic cyclohexene oxidation by tungstophospho(aqua)ruthenate via the fractional factorial design methodology†
Abstract
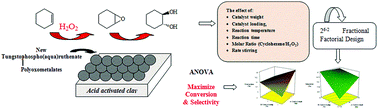
Keggin-type tungstophospho(aqua)ruthenate (PRuW) was synthesized and supported on acid activated montmorillonite under mild conditions. The prepared catalysts were characterized using X-ray fluorescence (XRF) spectroscopy, powder X-ray diffraction (PXRD), thermogravimetric analysis (TGA), surface area measurements and Fourier-transform infrared (FTIR) spectroscopy, and their catalytic activity in cyclohexene epoxidation was explored. X-ray diffraction indicated that PRuW was properly loaded on the acid activated montmorillonite support. Heterogenization of homogenous catalysts is really interesting as heterogeneous catalysts are recoverable. The optimum conditions were determined using fractional factorial design in which catalyst weight, catalyst loading, reaction temperature, time, reaction stirring and the molar ratio of cyclohexene/H2O2 were varied. A first order polynomial equation was developed to investigate the relationship between the responses and operational variables and the fitted model showed a good agreement between the predicted and actual responses. Furthermore, by employing regression analysis, an empirical model was developed to predict the recovery yield of the studied range of conditions.


 Please wait while we load your content...
Please wait while we load your content...