Synthesis and characterization of carbene-pyridyl anchoring Ru(ii) dyes with various binding functionalities for photoelectrochemical cells†
Abstract
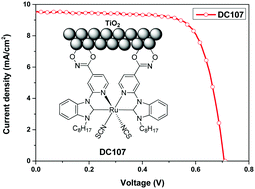
Ruthenium metal complexes consisting of various functionalized carbene-pyridyl anchoring ligands were synthesized, characterized, and tested for their power conversion capabilities. Carboxylate (DC101), hydroxamate (DC107), and acyl azide (DC108) groups were explored as anchors to identify a suitable and efficient binding functionality for the unique characteristic carbene-pyridyl ligand. The hydroxamate anchoring group was shown to be the most efficient anchoring group for the carbene-pyridyl ligand and the device based on dye DC107 exhibited the best efficiency (η) of 4.97% compared to those of the other prepared sensitizers. Electrochemical impedance spectroscopy studies on the devices based on these dyes justified their performances. This is the first case of an investigation utilizing σ electron donating carbene-pyridine as the anchoring ligand and it was found to be more suitable for use as an ancillary group.


 Please wait while we load your content...
Please wait while we load your content...