DFT study on the Au(i)-catalyzed cyclization of indole-allenoate: counterion and solvent effects†
Abstract
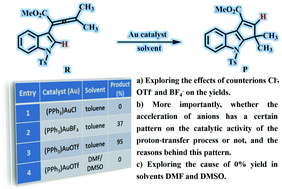
A computational study using the B3LYP density functional was carried out to explore the effects of counterions and solvents on the Au(I)-catalyzed cyclization reaction of indole-allenoate to form dihydrocyclopenta[b]indole derivatives. The optimal reaction path includes intramolecular cyclization and proton transfer steps. In the first process, the counterions Cl−, BF4− and OTf− act as hydrogen-bond acceptors to promote the intramolecular cyclization between the C1 and C5 atoms. In the proton transfer step, the anions greatly reduce the energy barrier of proton migration, in the form of a proton-transfer shuttle. More importantly, the Bronsted/Lewis basicity of the counterions (Cl− > OTf− > BF4−) turns out to be the primary reason for the difference in the counterion catalytic activity in the proton-transfer process. During the protonation of the counterion, the catalytic capacities of the counterions show significant differences according to the series Cl− > OTf− > BF4−, and the order of the catalytic ability of the counterions was found to be Cl− < OTf− < BF4− in the deprotonation of the counterion-H. Interestingly, the strong coordinating capability of the solvents (DMF and DMSO vs. PhCH3) was found to be another important factor that critically affects the reaction yield (0%, 0% and 95% yield, respectively). Overall, our calculations not only explain the experimental phenomena well, but also put forward some guidance and advice for the selection of counterions and solvents for transition metal-catalyzed reactions, including proton-transfer processes.


 Please wait while we load your content...
Please wait while we load your content...