Improving coloration time and moisture stability of photochromic viologen–carboxylate zwitterions†
Abstract
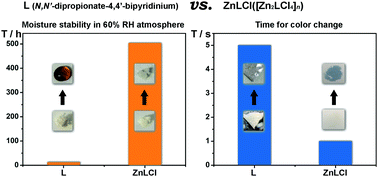
Viologen (N,N′-disubstituted bipyridinium) compounds are well known for their electron-transfer photochromic properties and many potential applications. However, viologen-based organic molecules, especially those with aliphatic hydrocarbon substituents, are inclined to be hygroscopic, and most of the known viologen compounds need dozens of seconds or even several minutes to exhibit photoinduced color change. The viologen–carboxylate zwitterion ligand, N,N′-dipropionate-4,4′-bipyridinium (L), is highly hygroscopic and its shortest coloration time is 5 s upon irradiation of a Xe lamp (ca. 110 mW cm−2). After coordination with ZnCl2, the obtained new compound [Zn2LCl4]n (ZnLCl) is highly stable in a wet environment (e.g. 60% relative humidity), and its shortest coloration time is 1 s upon irradiation of the same Xe lamp. X-ray diffraction analysis coupling with multiple experimental spectra (UV-vis, ESR, XPS, and IR) and density of states (DOS) calculations revealed that the photoinduced coloration of L and ZnLCl is contributed mainly by O → pyridinium and Cl → pyridinium electron transfer, respectively. The lower electronegativity of Cl over O and the shorter donor–acceptor separation may explain why ZnLCl has a shorter coloration time. Moreover, enhancement of molecular rigidity by the coordination structure may account for the improvement of moisture stability.


 Please wait while we load your content...
Please wait while we load your content...