Electrochemically growth-controlled honeycomb-like NiMoO4 nanoporous network on nickel foam and its applications in all-solid-state asymmetric supercapacitors†
Abstract
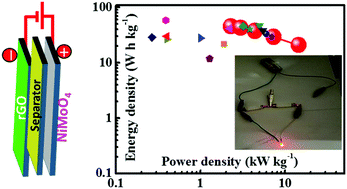
A honeycomb-like NiMoO4 nanoporous network electrode was synthesized on nickel foam using an electrodeposition method and used for the fabrication of asymmetric supercapacitors (ASCs). The growth process of the NiMoO4 nanostructure was controlled by varying the number of electrochemical cycles. The evolution of the NiMoO4 nanoflakes with the electrochemical cycles was confirmed by field emission scanning electron microscopy. The supercapacitive properties of NiMoO4 nanostructure were measured by cyclic voltammetry, galvanostatic charge discharge, and electrochemical impedance spectroscopy. The well-grown NiMoO4 nanoporous network exhibited a highest specific capacitance of 1475 F g−1 at a current density of 1 A g−1 in an aqueous KOH electrolyte with a good rate capability of 72.8% when the current density was increased 20 fold. The nanoporous network also exhibited a capacitive retention of 87.9% after 5000 charge–discharge cycles at a fixed current density of 20 A g−1. A NiMoO4//reduced graphene oxide (rGO)-based solid state ASC was fabricated using a poly(vinyl alcohol)–KOH gel electrolyte. The NiMoO4//rGO ASC delivered a maximum energy density of 45 W h kg−1 and a power density of 14.5 kW kg−1 as well as an electrochemical stability of 84% after 5000 cycles, highlighting the potential of NiMoO4//rGO ASCs for portable electronic applications.


 Please wait while we load your content...
Please wait while we load your content...