Removal of elemental mercury using titania sorbents loaded with cobalt ceria oxides from syngas
Abstract
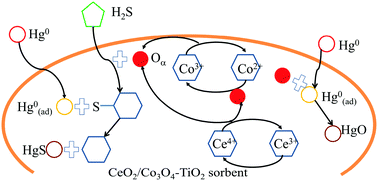
Titania sorbents loaded with cobalt ceria oxides prepared by a co-precipitation method were employed to remove elemental mercury (Hg0) from simulated syngas at low temperatures (80–240 °C). The effects of the Co/Ce mass ratio, reaction temperature and main syngas components (H2S, H2, CO) on Hg0 removal efficiency were investigated. BET, XRD and XPS were employed to characterize the sorbents. The results showed that up to 92% Hg0 removal efficiency could be obtained over Ce0.2Co0.1Ti at 120 °C. The remarkably high Hg0 removal efficiency of Ce0.2Co0.1Ti was attributed to the synergistic effect of cobalt and ceria. H2 and CO had a negligible effect on Hg0 removal under a N2 or H2S atmosphere. The characterization results indicated that the physical properties were not the main factors affecting the adsorbent activity. Moreover, XRD and XPS were used to elucidate the reaction mechanism between cobalt and ceria. Furthermore, Hg0 removal over Ce0.2Co0.1Ti was proposed to follow the Langmuir–Hinshelwood mechanism.


 Please wait while we load your content...
Please wait while we load your content...