2-Methylol-12-crown-4 ether immobilized PolyHIPEs toward recovery of lithium(i)
Abstract
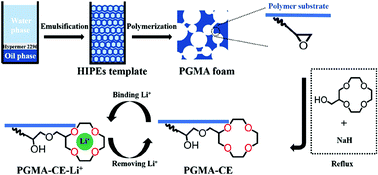
A facile strategy to fabricate crown ether (2-methylol-12-crown-4, 2M12C4) immobilized porous polymers (PGMA-CE) was reported toward lithium(I) (Li+) recovery. Macroporous polymer foam (polymeric glycidyl methacrylate, PGMA) with abundant epoxy groups was firstly prepared as a support using a high internal phase emulsion (HIPE) template. And then 2M12C4 was covalently attached onto the surface of PGMA to endow the PGMA-CE with good stability and high selectivity. PGMA-CE was characterized by SEM, FT-IR, XPS, and 13C NMR spectroscopy, and its porous structure and the successful introduction of 2M12C4 were confirmed. In batch mode experiments under optimum conditions, PGMA-CE exhibited fast adsorption kinetics (the equilibrium time was 3.0 h), and the kinetics data were well described using a pseudo-second-order model. A Langmuir-type monolayer adsorption was investigated, and the calculated maximum equilibrium adsorption capacity was 3.15 mg g−1. In addition, PGMA-CE displayed excellent selectivity for Li+ in the presence of multiple interfering ions (Na+, K+, Mg+, and Ca2+), and the selective separation factors (α) were above 4.75. Regeneration analysis showed that the adsorption capacity of PGMA-CE toward Li+ after five cycles was 91.8% of the first cycle.


 Please wait while we load your content...
Please wait while we load your content...