Conformation-induced light emission switching of N-acylhydrazone systems†
Abstract
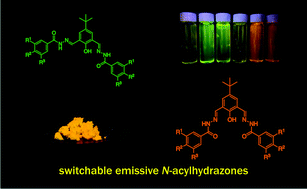
N-Acylhydrazones have been described as responsive compounds to a wide variety of triggers that are able to induce reversible structural changes. These reversible constitutional, conformational or configurational changes caused by physical or chemical stimuli are useful tools in designing molecular switches. We report herein bis-N-acylhydrazones as new switchable-light emissive molecules triggered by light and/or solvents. The emissive properties could be explained by phototautomerisation (Excited State Intramolecular Proton Transfer) and by formation of aggregates (Aggregation-Induced Emission). We investigated the influence of structural particularities brought in by electron-donor or electron-withdrawing substituents as well as the requirement of several hydrazone bonds in the molecular structure for the generation of light.


 Please wait while we load your content...
Please wait while we load your content...