Deep oxidative desulfurization of dibenzothiophene with {Mo132} nanoballs supported on activated carbon as an efficient catalyst at room temperature†
Abstract
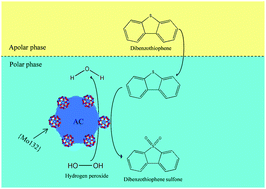
In this paper, the Keplerate nanoball iso-polyoxomolybdate {Mo132} supported on activated carbon (AC) has been synthesized and evaluated as a new, green and cost-effective catalyst for the oxidative desulfurization of a model fuel containing dibenzothiophene (DBT). The {Mo132}/AC catalysts, which were prepared with various {Mo132} contents, were characterized using FT-IR, XRD, SEM, EDX, ICP-OES, H2-TPR and nitrogen adsorption/desorption isotherms. Experimental evaluations showed that the catalyst was highly efficient in the removal of DBT using hydrogen peroxide (H2O2) as the oxidant, which could result in a sulfur removal of up to 99.5% (or even more than that) under optimum reaction conditions. The factors affecting the process including catalyst dosage, temperature, O/S molar ratio, reaction time and initial sulfur content were evaluated, and the optimum operating conditions were determined. In addition, the new catalyst was recoverable and the recovered {Mo132}/AC demonstrated a relatively similar catalytic activity to the fresh one. According to the results of GC-MS analysis, a sulfone species was found to be the only product of DBT oxidation by H2O2 over {Mo132}/AC. Moreover, a mechanism for the oxidative desulfurization of DBT by the new catalyst was proposed. The present study suggested that the {Mo132}/AC composite could be used as an efficient catalyst for the deep oxidative desulfurization and removal of refractory sulfur compounds in fossil fuels.


 Please wait while we load your content...
Please wait while we load your content...