Efficient bluish green electroluminescence of iridium complexes with good electron mobility†
Abstract
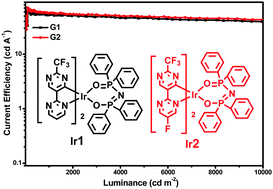
Two novel iridium(III) complexes (Ir1 and Ir2), synthesized using 2′-(trifluoromethyl)-2,5′-bipyrimidine and 5-fluoro-2′-(trifluoromethyl)-2,5′-bipyrimidine as the main ligands, and tetraphenylimidodiphosphinate (tpip) as an ancillary ligand, were investigated. The introduction of a nitrogen heterocycle and CF3 substituent improves the electron mobility of the Ir(III) complexes, which is beneficial for device performance. Both of the complexes emit bluish green photoluminescence with very high quantum efficiency yields (Ir1: λmax: 485/516 nm, ηPL: 89%; Ir2: λmax: 482/513 nm, ηPL: 95%) and good electron mobility. Organic light-emitting diodes (OLEDs) constructed with an ITO (indium tin oxide)/MoO3 (molybdenum oxide, 3 nm)/TAPC (di-[4-(N,N-ditolyl-amino)-phenyl]cyclohexane, 50 nm)/mCP (1,3-bis(9H-carbazol-9-yl)benzene, 5 nm)/Ir complex (6 wt%):PPO21 (3-(diphenylphosphoryl)-9-(4-(diphenyl-phosphoryl)phenyl)-9H-carbazole, 10 nm)/TmPyPB (1,3,5-tri(m-pyrid-3-yl-phenyl)benzene, 50 nm)/LiF (1 nm)/Al (100 nm) structure showed good device performances. Device G1 based on Ir1 showed a ηc,max value of 62.99 cd A−1 with an EQEmax value of 23.5%. Owing to the slightly higher PL efficiency and lower LUMO levels of Ir2, which are beneficial for electron injection, the device based on Ir2 displayed a slightly better performance with a ηc,max value of 71.18 cd A−1 and an EQEmax value of 27.7%. Even at a practical brightness of 1000 cd m2, values of 57.39 cd A−1 and 22.3% could still be reached.


 Please wait while we load your content...
Please wait while we load your content...