New bow-tie cationic carbosilane dendritic system with a curcumin core as an anti-breast cancer agent†
Abstract
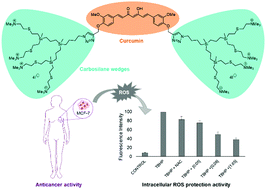
Though curcumin has been demonstrated as being highly cytotoxic towards various cancer cell lines, the major drawbacks for its use are the insolubility and instability in water, provoking low bioavailability. On the other hand, carbosilane dendritic systems with a cationic charge on the surface have been studied in different biomedical applications such as antibacterial, anticancer or as non-viral vehicles for the delivery of nucleic acids. In this study, we combine both systems by synthesizing a new “bow-tie” cationic carbosilane dendrimer where curcumin is located at the core. The resulting new system is completely water-soluble, maintains antioxidant properties and induces higher cytotoxicity against MCF-7 cancer cells compared with free curcumin. In addition, the effects on the cell cycle and apoptosis induction are evaluated by using flow cytometry analysis to obtain insights of the cytotoxic mechanism of action.


 Please wait while we load your content...
Please wait while we load your content...