S⋯S and S⋯P chalcogen bonding in solution: a cryospectroscopic study of the complexes of 2,2,4,4-tetrafluoro-1,3-dithietane with dimethyl sulfide and trimethylphosphine†
Abstract
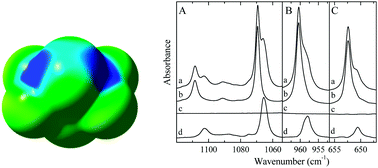
In this combined experimental and computational study the complexes formed between 2,2,4,4-tetrafluoro-1,3-dithiethane (C2F4S2) and the Lewis bases dimethyl sulfide (DMS) and trimethylphosphine (TMP) are studied using infrared spectroscopy of solutions in liquid krypton. For C2F4S2 and DMS, ab initio calculations yield two stable chalcogen bonded complex isomers, whereas for C2F4S2 and TMP a single chalcogen bonded complex is predicted. In the infrared spectra of solutions containing C2F4S2 and DMS, evidence for the presence of two complex bands suggesting the simultaneous occurrence of both calculated complex geometries and the lack of rapid interconversion between them is found for several vibrational modes. The average experimental complexation enthalpy for the 1 : 1 complexes derived from the van't Hoff isochores is −12.3(4) kJ mol−1. The calculated values obtained by combining a complete basis set extrapolation approach and corrections for zero-point vibrational, thermal and solvent influences are −17.3 and −15.8 kJ mol−1 for the first and second geometry, respectively. For the mixtures of C2F4S2 with TMP, single complex bands are found for all vibrational modes, with shifts corresponding well to those calculated for the chalcogen bonded complex. The experimental complexation enthalpy for the C2F4S2·TMP complex is −13.0(4) kJ mol−1, whereas the calculated value equals −16.1 kJ mol−1.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...