Co-sensitization of Ru(ii) complex with terthiophene-based D–π–π–A metal-free organic dyes for highly efficient dye-sensitized solar cells: influence of anchoring group on molecular geometry and photovoltaic performance†
Abstract
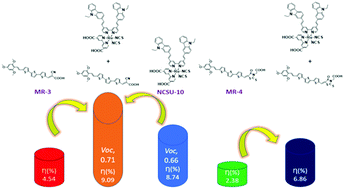
In this work, we studied the photovoltaic performance of two novel metal-free organic dyes (MR-3 and MR-4) with the molecular motif D–π–π–A carrying the same donor (trimethoxy benzene) and terthiophene (π) units but with different anchoring moieties. MR-3 and MR-4 displayed PCE of 4.54% and 2.38%, respectively. Furthermore, MR-3 co-sensitized with NCSU-10 exhibited improved efficiency of 9.09% than NCSU alone (8.74%). One of the merits in the studied structure motif D–π–π–A was modulating the molecular geometry of the sensitizers and eliminating the steric effect between the donor and π-spacer (terthiophene) to furnish a coplanar molecular structure and to maximize electronic conjugation. In addition, coupling-steric effect precludes effective conjugation and reduces light harvesting as it increases the band gap. To eliminate the steric effect of the aryl moiety at ortho position of 2,4,6-trimethoxyphenyl donor, another conjugated spacer, alkene (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), was inserted between the donor and the π-spacer (terthiophene), which maximized the electron conjugation throughout the coplanar system; the optimized molecular geometry of the dyes was calculated using DFT. Another molecular geometry feature that was studied was the influence of different anchoring groups on coplanarity of the dye structure and consequently the photovoltaic performance. In the case of MR-3, where the anchoring group is based on the cyanoacrylic group, the optimized geometry was found coplanar. However, when rhodanine moiety was used as an anchoring group, the calculated optimized geometry of the dye (MR-4) was not coplanar, which resulted in weak electronic coupling and inferior electronic injection into TiO2 conduction band edge. The inter-relationship between the optimized geometry of the dyes and their photovoltaic performance is discussed.
CH), was inserted between the donor and the π-spacer (terthiophene), which maximized the electron conjugation throughout the coplanar system; the optimized molecular geometry of the dyes was calculated using DFT. Another molecular geometry feature that was studied was the influence of different anchoring groups on coplanarity of the dye structure and consequently the photovoltaic performance. In the case of MR-3, where the anchoring group is based on the cyanoacrylic group, the optimized geometry was found coplanar. However, when rhodanine moiety was used as an anchoring group, the calculated optimized geometry of the dye (MR-4) was not coplanar, which resulted in weak electronic coupling and inferior electronic injection into TiO2 conduction band edge. The inter-relationship between the optimized geometry of the dyes and their photovoltaic performance is discussed.


 Please wait while we load your content...
Please wait while we load your content...