Tetranuclear iron carbonyl complexes with a central tin atom: relationship to iron carbonyl carbides†
Abstract
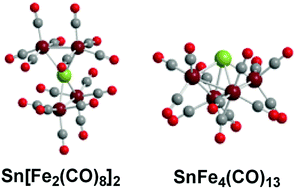
The two tetranuclear iron carbonyl systems EFe4(CO)n (E = Sn, C) containing central group 14 interstitial atoms differ in that spiropentane-like SnFe4(CO)16 has been synthesized in the tin system whereas the butterfly CFe4(CO)13, with three fewer carbonyl groups is the carbonyl-richest tetranuclear iron carbonyl carbide that has been synthesized. In order to clarify this point, the complete SnFe4(CO)n (n = 16, 15, 14, 13, 12) series has been studied by density functional theory for comparison with earlier similar studies on their CFe4(CO)n analogues. The experimentally observed spiropentane-like Sn[Fe2(CO)8]2 structure is found to be the lowest energy structure for the SnFe4(CO)16 system as it is for the experimentally unknown CFe4(CO)16 system. Loss of a CO group from Sn[Fe2(CO)8]2 joins the two Fe2(CO)8 units by a third Fe–Fe bond to give an SnFe4(CO)15 structure with a bonded four-atom Fe–Fe–Fe–Fe chain. Further CO loss from SnFe4(CO)15 adds a fourth Fe–Fe bond in the lowest energy SnFe4(CO)14 structure. The lowest energy SnFe4(CO)13 structure is analogous to that of the experimentally known iron carbonyl carbide CFe4(CO)13 with a central Fe4 butterfly having five Fe–Fe bonds. The energetics of CO dissociation from the EFe4(CO)n (E = C, Sn; n = 16, 15, 14, 13) species account for the experimentally observed differences between the systems with central tin and central carbon atoms. Thus for the tin systems the CO dissociation energy from SnFe4(CO)16 is relatively high at ∼50 kcal mol−1 consistent with its experimental observation as a stable species. However, for the tetranuclear iron carbonyl carbides CFe4(CO)n, the CO dissociation energies of the species with more than 13 CO groups are all very small or even negative suggesting CFe4(CO)13 to be the carbonyl-richest viable iron tetracarbonyl carbide consistent with experiment.


 Please wait while we load your content...
Please wait while we load your content...
