Complex salts of Pd(ii) and Pt(ii) with Co(ii) and Ni(ii) aqua-cations as single-source precursors for bimetallic nanoalloys and mixed oxides†
Abstract
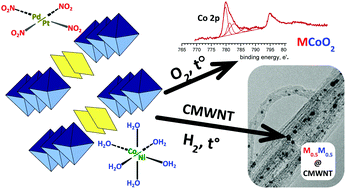
A series of highly soluble double complex salts [M′(H2O)6][M′′(NO2)4]·2H2O (M′ = Ni, Co; M′′ = Pd, Pt) have been synthesized, studied and used as single-source precursors for bimetallic nanoalloys and metal–oxide composites. According to the results of simultaneous thermal analysis-mass spectrometry (STA-MS), X-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (XRD), thermolysis of the compounds in diverse atmospheres (O2, He, and H2), delafossite-type mixed oxides M′′CoO2/(NiO + M′′O), metal–oxide composition M′O + M′′ and bimetallic solid solution M′0.5M′′0.5, respectively. In an H2 atmosphere an instant isolation of the equiatomic solid solution occurs at lower than 200 °C with simultaneous reduction of noble and ignoble metals. The bimetallic products can be superstructurally ordered at higher temperatures. The solubility of precursors in organic solvents allows carrying out an impregnation of the salts inside multiwalled carbon nanotubes; subsequent heating in H2 yields intercalated nanoparticles of ∼2–4 nm in size.


 Please wait while we load your content...
Please wait while we load your content...