Magnetic and thermodynamic properties of α, β, γ and δ-MnO2†
Abstract
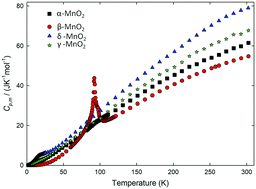
Four different structures of manganese dioxide particles with good dispersity were obtained using hydrothermal and co-precipitation methods. Magnetic measurement reveals that α-MnO2 exhibits a spin-glass like behavior below 50 K which is different from bulk α-MnO2. β-MnO2 and δ-MnO2 exhibit a transition from the paramagnetic to antiferromagnetic state at 92 K and 20 K, respectively, and γ-MnO2 retains the paramagnetic state during the temperature range of measurement. Besides, δ-MnO2 possesses the highest magnetization while β-MnO2 has the lowest magnetization. The thermodynamic properties of these manganese dioxides were further studied using heat capacity measurements, and the results confirmed magnetic transition at the corresponding temperatures revealed in the above magnetic measurements. Through fitting the heat capacity curve, the standard molar heat capacity, entropy and enthalpy at 298.15 K (0.1 MPa) and transition enthalpy were obtained in this work. Based on the characterization results, it is supposed that impurity ions/molecules and vacancies are the main factors that contribute to the magnetic performance of δ-MnO2, although the layer structure is thought to be an origin of ferromagnetic interaction.


 Please wait while we load your content...
Please wait while we load your content...