Novel functionalized organotellurides with enhanced thiol peroxidase catalytic activity†
Abstract
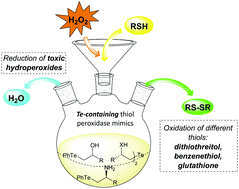
The thiol peroxidase-like activity of a series of novel functionalized tellurium containing catalysts has been investigated with different models. Dialkyl- and aryl-alkyl-tellurides, conveniently achieved through the ring opening of strained heterocycles, exhibited remarkable catalytic antioxidant activity, being able to reduce hydrogen peroxide in the presence of different thiols (benzenethiol, dithiothreitol and glutathione) under different conditions. The nature of the β-substituent strongly influenced the performances of the studied catalysts, thus giving useful criteria for the design of good synthetic mimics of glutathione peroxidase. The catalytic activity of functionalized organotellurides has been compared with that of their selenated analogues, showing as the latter behave as less efficient catalysts.


 Please wait while we load your content...
Please wait while we load your content...