A possible target: triple-bonded indium![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) antimony molecules with high stability†
antimony molecules with high stability†
Abstract
We have considered as a theoretical possibility the development of triple-bonded RIn![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR molecules bearing suitable substituents (R). Calculations have demonstrated that the RIn
SbR molecules bearing suitable substituents (R). Calculations have demonstrated that the RIn![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR molecules possessing smaller substituents (such as R = F, OH, CH3, H, and SiH3) cannot be stabilized. Only the triple-bonded R′In
SbR molecules possessing smaller substituents (such as R = F, OH, CH3, H, and SiH3) cannot be stabilized. Only the triple-bonded R′In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR′ molecules featuring sterically bulky groups (R′ = SiMe(SitBu3)2, SiiPrDis2, Tbt (= C6H2-2,4,6-{CH(SiMe3)2}3), and Ar* (= C6H3-2,6-(C6H2-2,4,6-i-Pr3)2)) are found to locate on the global minimum of the singlet potential energy surface and are thermodynamically stable. The valence-electron bonding model reveals that the bonding nature of R′In
SbR′ molecules featuring sterically bulky groups (R′ = SiMe(SitBu3)2, SiiPrDis2, Tbt (= C6H2-2,4,6-{CH(SiMe3)2}3), and Ar* (= C6H3-2,6-(C6H2-2,4,6-i-Pr3)2)) are found to locate on the global minimum of the singlet potential energy surface and are thermodynamically stable. The valence-electron bonding model reveals that the bonding nature of R′In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR′ can be represented as
SbR′ can be represented as 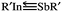 . Our computational investigations based on several theoretical methods (i.e., the charge decomposition analysis, the natural bond orbital analysis and the natural resonance theory) reveal that both the electronic and steric effects of bulkier substituent groups play important roles in making triple-bonded R′In
. Our computational investigations based on several theoretical methods (i.e., the charge decomposition analysis, the natural bond orbital analysis and the natural resonance theory) reveal that both the electronic and steric effects of bulkier substituent groups play important roles in making triple-bonded R′In![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SbR′ species synthetically accessible and isolable in a stable form.
SbR′ species synthetically accessible and isolable in a stable form.
![Graphical abstract: A possible target: triple-bonded indium [[triple bond, length as m-dash]] antimony molecules with high stability](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8NJ00549D&imageInfo.ImageIdentifier.Year=2018)


 Please wait while we load your content...
Please wait while we load your content...
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) antimony molecules with high stability
antimony molecules with high stability