Structural diversity in the products formed by the reactions of 2-arylselanyl pyridine derivatives and dihalogens†
Abstract
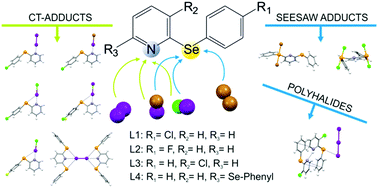
The reactivity of the 2-arylselanyl pyridine derivatives L1–L4 towards dihalogens X2 (X = I, Br) and interhalogens IX (X = Cl, Br) was studied in CHCl3 or CH3CN. The solid products obtained were structurally characterized and their nature points out the preference for CT spoke adducts and for seesaw insertion adducts to be formed at the N-donor and Se-donor site, respectively. DFT calculations were performed to provide a rationale for the structural diversity observed in the products obtained.
- This article is part of the themed collections: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST and The halogen bond: a new avenue in recognition and self-assembly


 Please wait while we load your content...
Please wait while we load your content...