NiO nanoparticle surface energy studies using first principles calculations†
Abstract
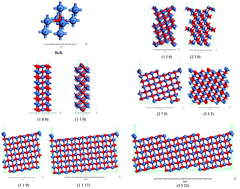
Understanding the correlations between active sites and surface energies of Miller index surfaces is of practical importance to get insights into catalytic efficiency. In this work, we investigate the effects of NiO nanoparticle surface energies on catalytic efficiency using first-principles calculations. This study reveals that nearly all high Miller index (HMI) surfaces can be grouped into three classifications of the corresponding low Miller index surfaces (1 0 0), (1 1 0), and (1 1 1) based on the similarity of the surface geometry and the number of broken bonds. Our experimental results revealed that higher surface energy particles which are dominated by the (1 1 1) or (1 1 0)-like HMI or the combination of (1 1 1) and (1 1 0)-like HMI surfaces lead to a higher catalytic performance. Therefore, guiding the design of highly efficient nanoparticle-based catalysts becomes operational and the possible catalytic efficiency of a nanoparticle-based catalyst could be theoretically assessed prior to experiments using surface energy calculations.


 Please wait while we load your content...
Please wait while we load your content...