Efficient hierarchically synthesized Fe2P nanoparticles embedded in an N,P-doped mesoporous carbon catalyst for the oxygen reduction reaction†
Abstract
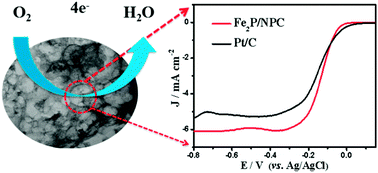
The construction of nonprecious metal catalysts for the oxygen reduction reaction (ORR) is highly desirable but remains as a key challenge for fuel cells and metal–air batteries. Herein, we designed and synthesized novel Fe2P nanoparticles embedded in an N,P co-doped mesoporous carbon catalyst (Fe2P/NPC) through direct pyrolysis of o-methylaniline in the presence of FeCl3·6H2O, H3PO4, silica nanospheres, and hexadecyltrimethyl ammonium bromide (CTAB) micelles. Transmission electron microscopy, Brunauer–Emmett–Teller (BET), X-ray diffraction and X-ray photoelectron spectroscopy measurements demonstrated that the obtained three-dimensional mesoporous Fe2P/NPC catalyst possessed a considerable specific surface area of 785 m2 g−1 with high contents of Fe2P nanoparticles, pyridinic N, and graphitic N species. Electrochemical evaluations showed that the formed Fe2P/NPC exhibited superior ORR performance to Pt/C in terms of a comparable onset potential (−0.01 V vs. Ag/AgCl electrode), about 20 mV higher half-wave potential, excellent stability and remarkable methanol tolerance in alkaline media. The synergistic effect of pyridinic N, graphitic N and Fe2P species together with its three-dimensional porous structure contribute to the impressive ORR performance. This research, most importantly, provides new clues to the rational development of cost-effective FePx-based catalysts for the ORR.


 Please wait while we load your content...
Please wait while we load your content...