Thermodynamic and spectroscopic study on the solvation and complexation behavior of Ln(iii) in ionic liquids: binding of Ln(iii) with CMPO in C4mimNTf2†
Abstract
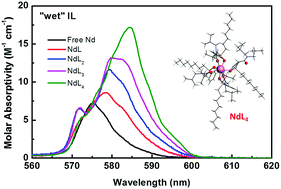
Fundamental coordination chemistry of metal ions in ionic liquids (ILs) is of great importance to extend the application of ILs in the area of metal separation. In this work, the solvation of representative trivalent lanthanides (Nd, Eu and La) and their complexation with a functional ligand, octylphenyl-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO, denoted as L), in the ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (C4mimNTf2) have been probed by spectroscopic, calorimetric and theoretical techniques. Absorption spectrophotometric titrations suggest that four successive Nd/CMPO complexes, NdLj3+ (j = 1–4), form both in “dry” (water content <250 ppm) and “wet” (water-saturated) ILs. However, the thermodynamic parameters vary distinctly in the two ILs. In “dry” IL, the complexation is stronger and overwhelmingly driven by exothermic enthalpies. In contrast, the complexation in “wet” IL is relatively weak and mainly driven by highly positive entropies. Comparisons between the fitted absorption spectra of Nd/CMPO complexes in “wet” IL and that of extractive samples from biphasic solvent extraction have clearly identified the extracted species as NdL43+ during the extraction. The formation of a 1 : 4 Ln/CMPO complex was further supported by DFT calculations and 31P-NMR results (La/CMPO). Additionally, luminescence emission spectra and lifetime of Eu(III) provide further evidence to illustrate the solvation and complexation behavior of Ln(III) in ILs. The results from this work shed light on how solvation affects the complexation of metal ions in ILs and how fundamental thermodynamic findings could help reveal the mechanism of biphasic extraction in real applications.


 Please wait while we load your content...
Please wait while we load your content...