Substituent influence in phenanthroline-derived ancillary ligands on the excited state nature of novel cationic Ir(iii) complexes†
Abstract
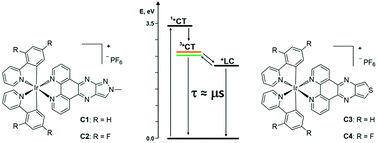
In the quest for coordination compounds with potential applications in energy conversion processes, a new series of four Ir(III) complexes (C1–4) of the type [Ir(R-ppy)2(Ln)](PF6), where R-ppy = 2-phenylpyridine (ppy) or 2,4-difluorophenylpyridine (F2-ppy) and Ln = 1-methyl-1H-pyrazole[3′,4′:5,6]pyrazino[2,3-f][1,10]phenanthroline (L1) or thieno[3′,4′:5,6]pyrazino[2,3-f][1,10]phenanthroline (L2) has been synthesized. The photophysical properties of these compounds have been thoroughly characterized by both steady-state and time-resolved spectroscopic techniques, pointing out a complex interplay between excited states of different nature that plays a crucial role in the deactivation processes. In the case of complexes C1–2 that feature the same L1 ancillary ligand, the lowest excited states at room temperature are characterized by an admixture between the 3MLCT/3LLCT and 3LC states, with an almost pure 3LC character in C2. For C3–4, the admixture among charge-transfer and ligand-centred states is negligible, due to the appreciably low energy of the LC one, which, however, plays a non-innocent role in the deactivation pathway of the triplet charge-transfer emissive states of complexes C3–4. This work thus highlights the importance of a detailed comprehension of the photophysical properties of Ir(III) complexes in view of their use in energy transformation systems.


 Please wait while we load your content...
Please wait while we load your content...