An iron hydroxyl phosphate microoctahedron catalyst as an efficient peroxidase mimic for sensitive and colorimetric quantification of H2O2 and glucose
Abstract
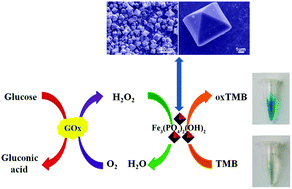
A colorimetric assay for H2O2 that is based on the use of iron hydroxyl phosphate (Fe3(PO4)2(OH)2) microoctahedrons, which are shown to be efficient peroxidase mimics, has been described. The microoctahedrons were prepared via a one-pot hydrothermal method. They catalyze H2O2 reduction and 3,3′,5,5′-tetramethylbenzidine (TMB) oxidation to produce a blue product whose color can be observed visually and measured using spectrophotometry, best at 652 nm. The method was applied for the quantification of H2O2 with a limit of detection (LOD) of 0.17 μM. The scope of the method was further extended to the determination of glucose by using the enzyme glucose oxidase which catalyzes glucose oxidation to produce gluconic acid and H2O2 which was quantified as described above. The response is linear in the 5–100 μM glucose range, and the LOD is 1.2 μM. This work not only reports a new kind of peroxidase mimic, but also develops a simple, robust and sensitive colorimetric sensing platform for H2O2 and glucose detection in biomedicine and food industry.


 Please wait while we load your content...
Please wait while we load your content...