Development of a new diffusive gradient in the thin film (DGT) method for the simultaneous measurement of CH3Hg+ and Hg2+
Abstract
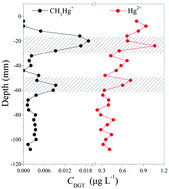
In this study, a Tulsion® CH-95 binding gel-based diffusive gradient in the thin film (TCH DGT) method was developed for the simultaneous measurement of CH3Hg+ and Hg2+. The binding kinetics of CH3Hg+ and Hg2+ in a mixed solution indicated rapid uptake to the TCH gel. The Hg2+ exhibited slower absorption rates compared to the CH3Hg+, which did not affect the DGT uptake overall. Stable elution efficiencies were obtained for CH3Hg+ and Hg2+ using a mild reagent containing 0.1 M HCL and 2% thiourea. The diffusion coefficients of CH3Hg+ and Hg2+ in agarose gel were calculated on the basis of the time-series deployment method and were 9.94 (×10−6 cm2 s−1, 25 °C) and 7.65 (×10−6 cm2 s−1, 25 °C), respectively. The DGT uptakes were independent of pH for CH3Hg+ (pH 4.10–8.10) and Hg2+ (pH 4.10–10.03), with no effect of the ionic strengths between 0.1–1000 mM NaCl. The capacity of TCH DGT for CH3Hg+ and Hg2+ according to linear and theoretical responses were calculated to be 33.97 μg cm−2 and 30.34 μg cm−2, respectively. The performance of this technique was further tested in sediment. Two distinct peaks of CH3Hg+ and Hg2+ appeared at similar sediment depths, reflecting that the localized remobilization of CH3Hg+ and Hg2+ may occur through similar mechanisms. This confirms that the TCH DGT is a useful in situ monitoring tool.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...