Base-promoted ring opening of 3-chlorooxindoles for the construction of 2-aminoarylthioates and their transformation to quinazolin-4(3H)-ones†
Abstract
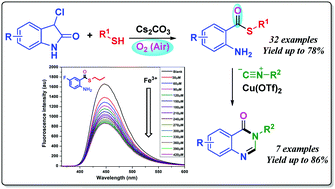
An unprecedented methodology for the preparation of diverse S-alkyl 2-aminoarylthioates has been developed based on cesium carbonate-promoted direct ring opening of 3-chlorooxindoles with thiols. This novel protocol proceeds via cascade sulfenylation, aerial oxidation, C2–C3 bond cleavage, and decarboxylation. The resulting products from this transformation exhibit potential for application as fluorescent sensors for the detection of Fe3+ ions. In addition, this methodology provides a concise pathway for the construction of a variety of biologically interesting quinazolin-4(3H)-ones in good yields.


 Please wait while we load your content...
Please wait while we load your content...