Photocleavable antimicrobial peptide mimics for precluding antibiotic resistance†
Abstract
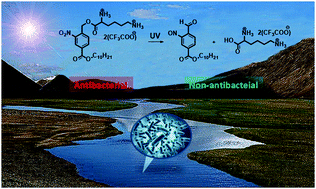
Cationic amphiphiles featuring a lysine-based hydrophilic moiety, an alkyl chain-based hydrophobic moiety, and an o-nitrobenzyl group-based linker were constructed facilely, which show alkyl chain-dependent antibacterial activity against both Gram-positive and Gram-negative bacteria, low cytotoxicity toward mammalian cells, and UV-cleavable properties, representing a novel type of environmental accumulation-free antimicrobial peptide mimic.


 Please wait while we load your content...
Please wait while we load your content...