Synthesis, structure and proton conductivity of 2,4,5-trimethylbenzenesulfonic acid dihydrate†
Abstract
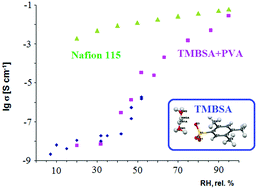
2,4,5-Trimethylbenzenesulfonic acid dihydrate was isolated in the monocrystalline state. Crystal and molecular structures of the compound were determined by X-ray diffraction analysis and vibrational spectroscopy. The compound crystal system is monoclinic: a =7.7445(3) Å, b = 7.5360(3) Å, c = 9.9477(4) Å, α = γ = 90°, β = 92.123(4)°, V = 580.17(4) Å3, P21, Z = 2, R1 = 0.0323, dcalc. = 1.353 g cm−3, T = 150 K. A 2D hydrogen bond network formed of a dioxonium ion and oxygen atom of a sulfogroup was identified in the crystal structure. IR absorption spectra, Raman spectra and ATR IR spectra of 2,4,5-trimethylbenzenesulfonic acid dihydrate and deuterodihydrate have been obtained in the region 50–4000 cm−1. It was found that the proton and deuterium in the H5O2+ and D5O2+ cations shifted from the midpoint of the strong hydrogen bond toward one of two water molecules according to the scheme O⋯H+⋯O → O⋯H+–O and O⋯D+⋯O → O⋯D+–O. Spectroscopic data concerning dioxonium ion formation show satisfactory agreement with X-ray diffraction analysis. The effect of environment humidity on the state of 2,4,5-trimethylbenzenesulfonic acid dihydrate aggregation was investigated. It was shown that the number of water molecules increases up to 2.5 per sulfogroup in the 7–84 rel.% air humidity interval through additional water molecules adsorption. A further humidity increase led to compound deliquescence. The proton conductivity as measured by impedance spectroscopy is 10−9–10−5 S cm−1 in the 7–58 rel.% humidity interval at 298 K with a [H2O]/[SO3−] = 2.2 ratio at maximum conductivity.


 Please wait while we load your content...
Please wait while we load your content...