Enantiopure Schiff bases of amino acid phenylhydrazides: impact of the hydrazide function on their structures and properties†
Abstract
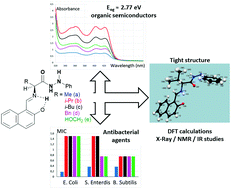
A rapid and eco-friendly synthesis of new enantiopure Schiff bases 5a–e was performed from various α-amino acid phenylhydrazides and 2-hydroxynaphthaldehyde using microwave or ultrasound irradiation in yields ranging from 57 to 75%. These imines were characterized using 1H & 13C NMR, FTIR, mass and UV-vis spectroscopies. In addition, the solid state molecular structure of 5b was determined by single crystal X-ray diffraction which shows that this compound adopts the zwitterionic form, crystallizing in the non-centrosymmetric orthorhombic system with the space group P212121. DFT calculations show that the BMK/6-31G(2df,2pd) method in the gas phase is well adapted to describe the geometry of the solid state. Theoretical results corroborate the ionic character of this species in concordance with spectroscopic results and lead to a new thin description. The Schiff bases 5a–e behave as organic semiconductors with Eog ≈ 2.77 eV. The in vitro antibacterial study showed that these molecules exhibited various levels of antibacterial effect against all of the tested bacterial strains.


 Please wait while we load your content...
Please wait while we load your content...