Electrodeposition of Ni–Mo–rGO composite electrodes for efficient hydrogen production in an alkaline medium†
Abstract
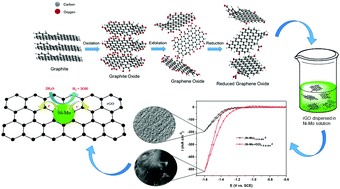
The mechanism and kinetics of the hydrogen evolution reaction (HER) on Ni–Mo–rGO composite electrodes in 1.0 M KOH solution were investigated by cyclic voltammetry (CV), chronopotentiometry (CP) and potentiodynamic polarization techniques. Ni–Mo–rGO composite coatings were deposited on a copper substrate by an electrodeposition method at a current density (c.d.) ranging from 1.0 to 4.0 A dm−2. The change in surface morphology and chemical composition was characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, energy dispersive X-ray (EDX) analysis and X-ray photoelectron spectroscopy (XPS). It was shown that the carbon content of the composite coatings was affected by c.d. With the increase in the carbon content in the Ni–Mo–rGO composite coatings, the onset potential was decreased and the exchange current density was increased during the HER. The minimum onset potential and maximum exchange current density of Ni–Mo–rGO composite coatings for the HER were −401.6 mV and 4.31 μA cm−2. The best composite coating exhibited a maximum peak current density of −0.517 A cm−2 at −1.6 V, which is approximately 3 times better than that of the binary Ni–Mo alloy, indicating the best activity for hydrogen production. The potentiodynamic polarization measurements revealed that composite coatings are much more resistant to corrosion than binary alloy coatings.


 Please wait while we load your content...
Please wait while we load your content...