Zn–Fe-layered double hydroxide intercalated with vanadate and molybdate anions for electrocatalytic water oxidation†
Abstract
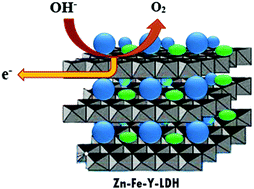
In this study, vanadate and molybdate anions were intercalated into Zn–Fe layered double hydroxides (LDHs) by co-precipitation methods to obtain VO4-LDH and MoO4–LDH nanohybrids. Powder X-ray diffraction (XRD), Fourier transform infrared spectra (FT-IR), transmission electron microscopy (TEM), and X-ray energy dispersive spectroscopy (EDS) indicate the successful intercalation of vanadate and molybdate anions. According to the results obtained from TEM and XRD, LDHs have a size in the nanometer scale with a plate-like morphology and high crystalline quality. Moreover, the LDHs were used as an efficient electrocatalyst material for water oxidation in alkali solution. In electrochemical water splitting, Zn–Fe–VO4 shows a superior OER performance compared to the bare glassy carbon electrode. Zn–Fe–VO4-LDH exhibits good OER activity, which is expressed as a low onset overpotential, small Tafel slope, and large exchange current density. At the overpotential of 0.46 (V vs. SCE), the current density of the Zn–Fe–VO4-LDH nanosheets is about 188.43 mA cm−2, which is much higher than that of the Zn–Fe–MoO4–LDH and Zn–Fe–NO3–LDH nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...