Facile synthesis of electrostatically anchored Nd(OH)3 nanorods onto graphene nanosheets as a high capacitance electrode material for supercapacitors†
Abstract
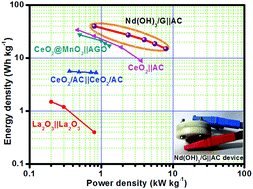
Neodymium hydroxide nanorods [Nd(OH)3] are developed by a facile chemical precipitation method without any surfactants/templates at ambient temperature. The neodymium hydroxide nanorods–graphene [Nd(OH)3/G] nanohybrid is prepared by a simple solvothermal reduction process using Nd(OH)3/GO in a mass ratio of 1 : 0.5 in dimethylformamide for 7 h. This new Nd(OH)3/G nanohybrid can be used as an electrode material for supercapacitors; it exhibits good capacitive behaviour, with a specific capacitance of 820 F g−1 at 1 A g−1. The nanohybrid exhibits a capacitance retention of 96% even after 3000 continuous charge–discharge cycles. This excellent electrochemical behaviour is mainly attributed to the synergetic effect of Nd(OH)3 and graphene. The asymmetric supercapacitor (ASC) device is denoted as Nd(OH)3/G‖AC; a poly(vinylidene fluoride) electrospun membrane soaked in 6 M KOH was used as a separator as well as the electrolyte. The ASC device functions in an optimized potential window of 1.6 V with an energy density of 40 W h kg-1. Furthermore, the ASC device exhibits excellent capacitance retention of 85.3% with a Coulombic efficiency of 97% even after 5000 cycles. This new hybrid electrode material shows impressive performance and can be used as an electrode material for asymmetric supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...