Synthesis of novel 1,2,3-triazole based artemisinin derivatives and their antiproliferative activity
Abstract
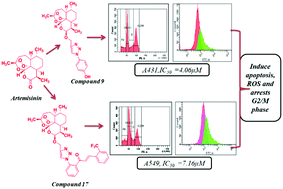
Two series of novel 1,2,3-triazole based artemisinin derivatives were designed, synthesized via a copper(I)-catalyzed azide alkyne cycloaddition (CuAAC) reaction and investigated for their antiproliferative activity by MTT assay against various human cancer cell lines. In series 1, compounds 9, 17, 18, and 22 showed better antiproliferative activity against all the tested cell lines as compared to dihydroartemisinin (DHA, 5). Compound 9 and compound 17 were the most active, with an IC50 range from 4.06 to 36.65 μM and 7.16 to 28.21 μM, respectively. Compound 9 showed potent antiproliferative activity against the A431 cell line with an IC50 of 4.06 μM and compound 17 displayed potent activity against the A549 cell line with an IC50 of 7.16 μM. In series 2, compounds 24, 27 and 28 showed better activity than the other derivatives. Active compounds 9 and 17 showed significant (p < 0.05) cell cycle arrest at the G2/M phase and apoptosis in skin and lung cancer cells. These molecules significantly (p < 0.05) induce ROS formation in the tested cell lines. Furthermore the toxicity study on human erythrocytes revealed that these molecules are non-toxic even at the highest tested concentration (100 μg mL−1). Some 3α-hydroxydeoxydihydroartemisinin-triazole derivatives (11a, 14a, 15a, 17a–22a) were also synthesized along with their peroxy counterparts. The antiproliferative activity results revealed that, except for compound 11a, all the compounds with peroxy functionality are more active than their 3α-hydroxydeoxy counterparts.


 Please wait while we load your content...
Please wait while we load your content...