Structure, magnetic properties and DFT calculations of azido-copper(ii) complexes with different azido-bonding, nuclearity and dimensionality†
Abstract
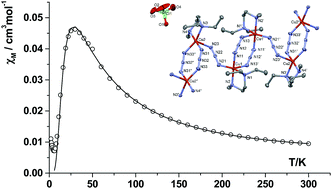
Four bridged-azido Cu(II) complexes were synthesized and structurally characterized: [Cu2(Et3en)2(μ1,1-N3)2(N3)2] (1), [Cu2(ip2en)2(μ1,1-N3)2(N3)2] (2), catena-[Cu2(Et2Meen)2(μ1,3-N3)3]ClO4 (3) and catena-[Cu(ambza)(μ1,1,3-N3)2] (4), where Et3en = N,N,N′-triethyl-1,2-diaminoethane; ip2en = N,N′-diisopropyl-1,2-diaminoethane; Et2Meen = N,N-diethyl-N′-methyl-1,2-diaminoethane; and ambza = 2-aminobenzylamine. Single crystal X-ray crystallography revealed that complexes 1 and 2 are dinuclear with doubly bridged μ1,1-N3, whereas complexes 3 and 4 constitute polymeric species with an alternative sequence of single and double μ1,3-N3 bridges for 3 with a 1D chain, and triple μ1,1,3-bridging azide in 4 with a 2D extended structure. Variable temperature magnetic susceptibilities over the temperature range of 2–298 K revealed a weak antiferromagnetic coupling (J = −1.8 cm−1) for 1, and a ferromagnetic coupling with J values of 7.0 and 7.7 cm−1, respectively, for complexes 2 and 4, respectively. Compound 3 shows a new topology for Cu(II) alternating chains [Cu(1)(μ1,3-N3)2Cu(1′)]-(μ1,3-N3)-[Cu(2′)(μ1,3-N3)2Cu(2′′)]-(μ1,3-N3)-[Cu(1)(μ1,3-N3)2Cu(1′)]-… Three different coupling constants were obtained through a fitting procedure and by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...