End-on cyanate or end-to-end thiocyanate bridged dinuclear copper(ii) complexes with a tridentate Schiff base blocking ligand: synthesis, structure and magnetic studies†
Abstract
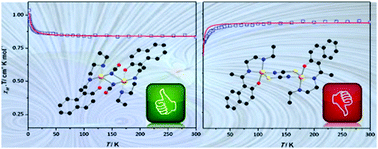
Two dinuclear copper(II) complexes, [Cu2(L)2(μ1,1-NCO)2] (1) and [Cu2(L)2(μ1,3-NCS)2]·H2O·DMF (2), have been synthesized using a tridentate N2O donor Schiff base ligand (HL), [1((2-(ethylamino)ethylimino)methyl)naphthalen-2-ol], and characterized by elemental analysis, spectral study and X-ray crystallography. Both complexes are centrosymmetric dimers in which square pyramidal copper(II) centres are connected by pseudo-halides: end-on cyanate in 1 and end-to-end (EE) thiocyanate in 2. Variable temperature (2–300 K) magnetic susceptibility measurements indicate the presence of ferromagnetic exchange coupling between copper(II) centres in complex 1 (J = 0.97 cm−1), and antiferromagnetic exchange coupling in 2 (J = −0.6 cm−1).


 Please wait while we load your content...
Please wait while we load your content...