Pyridine/pyridinium symmetrical bisamides as functional materials: aggregation, selective sensing and drug release†
Abstract
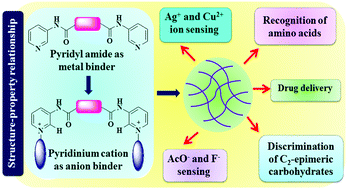
Pyrrole and furan-based pyridine/pyridinium bisamides 1–4 have been designed and synthesized with a view to establishing new supramolecular gelators. The neutral gelators 1 and 2 were explored for cation binding. While the furan analogue 1 forms gel in the presence of Ag+ and Cu2+ ions in DMSO : H2O (1 : 1, v/v), the pyrrole conjugate 2 exhibits metallogel formation selectively with Ag+ ions and corroborates the role of the heteroatom in the spacer that modulates the self assembly behavior of the gelators. As an application, the 1-Cu gel selectively distinguishes thiol-based amino acids from a series of other amino acids as well as effectively discriminates D-glucose from its C2-epimer D-mannose by exhibiting gel to sol transformation. Additionally, encapsulation and subsequent release of the chemopreventive drug curcumin has been studied using the 1-Cu gel. Placement of a cholesterol unit onto the structures 1 and 2 resulted in charged structures 3 and 4 of which compound 4 produced an unambiguous visual readout of F− and AcO− ions by showing gel to sol transition.


 Please wait while we load your content...
Please wait while we load your content...