Evidence for the electrochemical production of persulfate at TiO2 nanotubes decorated with PbO2
Abstract
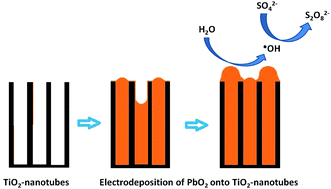
It is well known that PbO2-based electrodes are considered to be non-active anodes, producing higher concentrations of hydroxyl radicals in aqueous solutions, and consequently, favouring the electrochemical degradation of organic pollutants. However, no evidence has been reported on the production of persulfates using this kind of electrode in sulphate aqueous solutions. For this reason, the aim of this work is to prepare (by an electrochemical procedure (anodization and electrodeposition)) and characterize (by X-ray diffraction, scanning electron microscopy, and potentiodynamic measurements) Ti/TiO2-nanotubes/PbO2 disk electrodes (with a geometrical area of 65 cm2) in order to evaluate the electrochemical production of persulfate using Na2SO4 solution as the support electrolyte and applying current densities of 7.5 and 60 mA cm−2, as well as the influence of the electrosynthesis of hydroxyl radicals, in concomitance. The results clearly showed that significant production of hydroxyl radicals and persulfate is achieved at the Ti/TiO2-nanotubes/PbO2 surface, but this depends on the current density. The production of ˙OH at the Ti/TiO2-nanotubes/PbO2 surface in Na2SO4 solution was confirmed by a RNO spin trapping reaction. The results were compared with those of a Ti/Pt electrode in order to understand the effect when a lower amount of ˙OH is produced at the active anode surface. Based on the results, the Ti/TiO2-nanotubes/PbO2 anode could exhibit good electrocatalytic properties for environmental applications involving persulfate oxidants.


 Please wait while we load your content...
Please wait while we load your content...