Design, synthesis and photophysical studies of styryl-based push–pull fluorophores with remarkable solvatofluorochromism†
Abstract
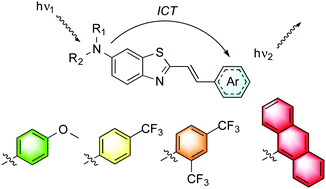
A library of 20 styryl-based push–pull dyes derived from 6-amino substituted benzothiazoles were prepared by an efficient and practical synthetic route from low-cost starting materials. The dyes were firstly designed to present an effective anchoring site for subsequent conjugation. A series of aryl scaffolds, from substituted phenyl rings containing electron donating and withdrawing groups to polycyclic aromatic derivatives, were screened. The inductive effect of N-alkyl substituted benzothiazoles was also explored for three different arrangements. The investigation of the structure–photophysics relationship was performed by UV-vis absorption and steady-state fluorescence emission measurements in solution and by TD-DFT calculations. The dyes presented high brightness, absorption bands in the visible range (∼370–453 nm) and large solvatofluorochromism comprising all the visible spectrum, as a consequence of the strong intramolecular charge transfer (ICT) nature of their excited state.


 Please wait while we load your content...
Please wait while we load your content...